Picture-crosslinking of the 3D bioprinting course of supported with an optical fiber
Generally, photo-crosslinkable bioinks, comparable to methacrylated hydrogels based mostly on gelatin, collagen, hyaluronic acid, and decellularized extracellular matrix, have been extensively utilized in fabricating cell-loaded constructs owing to their excellent cell-encapsulating talents and controllable mechanical properties that may be successfully achieved utilizing numerous photo-crosslinking processes9,19,20,21,22. When photo-crosslinkable bioinks are mixed with a basic bioprinting course of, multilayered cell-laden constructs with customizable pore geometries will be fabricated efficiently. Nevertheless, the exact and steady formation of cell constructs fabricated utilizing bioprinting supplemented with a photo-crosslinking course of critically relies on the crosslinking strategy employed. Of the assorted crosslinking methods which might be mixed with bioprinting, essentially the most breakthrough technique is the “in situ photo-crosslinking technique” executed utilizing a clear hydrophobic silicone nozzle, which might decrease the shear stress of a flowing photo-crosslinked-bioink8. When the bioink passes by way of the nozzle, UV mild will be straight uncovered to the clear nozzle to crosslink the flowing photo-crosslinkable and low-viscosity bioink. This technique is of immense significance in that it successfully and stably crosslinks low-viscosity bioinks to achieve mechanically steady cell-laden struts. Nevertheless, the in situ photo-crosslinking course of requires a specifically designed clear and hydrophobic photo-permeable nozzle. Moreover, as a result of the floor of the cell-laden struts will be extra actively crosslinked than the core area of the printed struts, the adhesive property between the struts will be comparatively poor after fabricating 3D cell buildings, leading to a low 3D structural shape-ability.
To beat the shortcomings of in situ crosslinking of photo-crosslinkable bioinks, we developed a brand new crosslinking technique utilizing an optic-fiber-assisted 3D bioprinting (OAB) course of that was fully completely different a traditional bioprinting (CB) utilizing an in situ crosslinking course of (Fig. 1a–c). To evaluate the efficiency of the OAB course of, we chosen a photo-crosslinkable Gelma-based bioink laden with C2C12 myoblasts with a density of 1 × 107 cells/mL. It’s because Gelma will be thought of a typical photo-crosslinkable bioink with biocompatible and biodegradable properties, and it may be extensively utilized in tissue engineering scaffolds, drug supply methods, and therapeutic substrates of wound areas23,24,25. To effectively crosslink the bioink, an optical fiber was embedded in a basic printing nozzle, and by controlling the depth of a UV mild supply (UV-spot head, proven in Fig. 1d), we might receive a mechanically steady and biologically secure 3D cell-laden assemble, as introduced within the optical and stay (inexperienced)/lifeless (crimson) photographs in Fig. 1a and the optical picture of the printed Gelma mesh-structure (Fig. 1c). Determine 1a, c presents an optical picture of UV mild as noticed by way of the optical fiber inserted throughout the nozzle. As well as, to keep away from an increase within the temperature of the bioink by the optical fiber, a cooling water jacket (4 °C) was set across the printing nozzle (Fig. 1c, d). In comparison with typical post-crosslinking, the proposed OAB course of can receive cell-laden 3D buildings with intrinsic morphologies, as demonstrated by the improved circularity values (Supplementary Fig. 1). Moreover, the OAB technique can fabricate a sensible multilayered 3D construction in distinction to CB, owing to enhanced strut-to-strut adhesion.
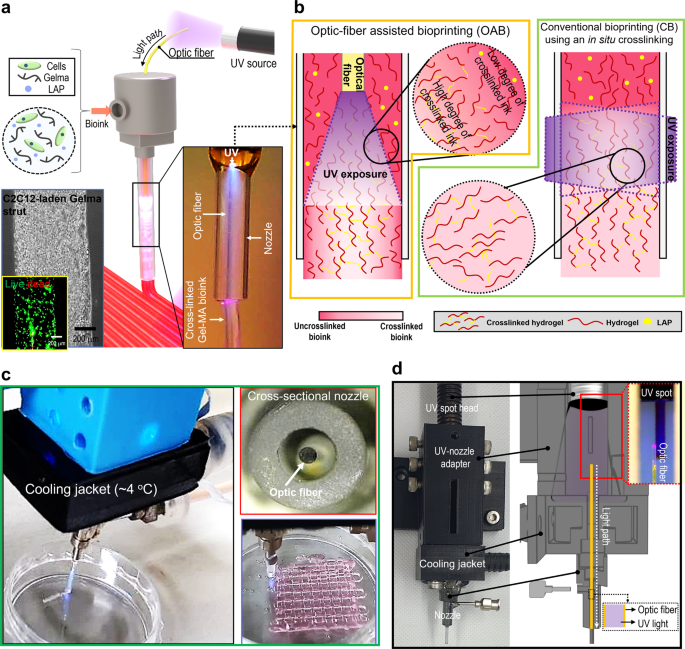
a Schematic of an optic-assisted bioprinting course of (OAB) utilizing an optical fiber positioned inside a nozzle of a printer, and optical and stay (inexperienced)/lifeless (crimson) photographs of the printed C2C12-laden Gelma strut. b Schematics of a photo-crosslinked hydrogel inside a nozzle processed with OAB and CB course of. c, d OAB system related with a water-cooling jacket, cross-sectional printing nozzle picture, and an optical picture of a printed mesh cell-laden Gelma construction.
Number of acceptable crosslinking and printing situations for the OAB course of
To watch the impact of the OAB course of on the photo-crosslinking means of the Gelma bioink, 5 wt% Gelma was used. Determine 2a illustrates the storage modulus (G′) of the Gelma bioink within the time-sweep mode earlier than and after UV publicity (UV dose = 120 mJ/cm2 for 10 s). As anticipated, the stiffness of Gelma uncovered to UV mild was considerably enhanced after UV publicity.
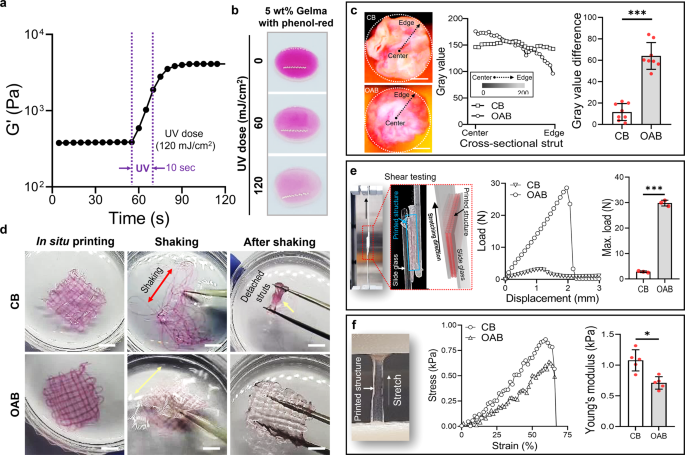
a Storage modulus (G′) of 5 wt% Gelma obtained from time-sweep assessments carried out earlier than and after UV publicity (UV dose = 120 mJ/cm2 for 10 s). b Coloration change within the combination of 5 wt% Gelma and phenol-red at numerous UV doses. c Cross-sectional optical photographs of the printed Gelma/phenol-red struts fabricated utilizing typical bioprinting (CB) and OAB processes, the distribution of the grey worth utilizing the cross-sectional photographs for radius, and the distinction of grey worth on the heart and edge areas. d Optical photographs of printed Gelma mesh buildings after in situ printing, throughout shaking, and after shaking as an example the structural stability. e Shear testing of the printed mesh buildings processed with CB and OAB to watch the adhesive properties between the struts. f Tensile stress–pressure curves and Younger’s modulus of the printed Gelma buildings. All knowledge are introduced as imply ± SD. The p values had been calculated by scholar’s t take a look at (n = 3/group; *p < 0.05; **p < 0.01; ***p < 0.001). Scale bar, 200 µm (c); 1 mm (d).
To watch the impact of photo-crosslinking produced by the optical fiber positioned within the inside of the nozzle on the crosslinking distribution over the cross-sectional space of a Gelma strut, we measured the distribution of the red-color depth of phenol-red, which differed relying on the photo-crosslinking diploma. Determine 2b depicts the assorted red-color intensities for the combination of 5 wt% Gelma/phenol-red (150 mg/L) at completely different UV doses (0, 60, and 120 mJ/cm2), indicating that the red-color pale into white at an growing UV dose. Determine 2c illustrates two cross-sectional photographs of Gelma struts that had been crosslinked utilizing the CB and OAB processes, respectively. Within the printing/crosslinking processes, the dimensions of the printing nozzle, nozzle velocity, pneumatic stress, and UV dose had been 700 μm, 1 mm/s, 120 ± 10 kPa, and 120 mJ/cm2, respectively.
The distribution of the crimson colour for the photo-crosslinked gelatin struts fabricated utilizing the CB and OAB strategies is illustrated in Fig. 2c. As indicated within the outcomes, the cross-sectional distribution of grey colour illustrating the diploma of photo-crosslinking was completely different for every technique. For the CB course of, the relative grey colour distribution within the cross-sectional area of the Gelma strut was related on the radius, whereas for the Gelma strut processed with the OAB, photo-crosslinking was comparatively greater within the core area than within the shell area (the graph of the grey worth distinction in Fig. 2c). From these outcomes, we are able to count on that the comparatively low diploma of crosslinking within the shell area of the strut can have an effect on the 3D structural formation of multilayered printed filaments. Determine 2nd illustrates printed mesh buildings fabricated utilizing the CB and OAB processes (5 wt% Gelma, UV dose = 120 mJ/cm2, nozzle dimension = 700 μm, nozzle shifting velocity = 2 mm/s, and pneumatic stress = 120 ± 10 kPa). After fabricating the Gelma buildings, the printed buildings had been shaken. As proven within the optical photographs, the 3D mesh construction was destroyed within the Gelma struts fabricated utilizing the CB course of, whereas the Gelma mesh construction fabricated utilizing the OAB course of was stably sustained in its initially designed form. This consequence signifies that the gelatin struts printed utilizing the OAB course of adhered nicely to one another to maintain the 3D structural form.
To guage the adhesive drive between the Gelma struts, we measured the displacement versus load for shear testing (Fig. 2e). Consequently, the adhesive power between the Gelma struts and the utmost load of the interfacial drive between the Gelma struts fabricated utilizing the OAB course of had been considerably greater than these for the Gelma struts processed utilizing the CB course of. This indicated that the 3D structural formations consisting of multilayered struts printed with the OAB course of will be far more effectively obtained than these printed with the CB course of. Nevertheless, for the tensile stress–pressure curves, the construction processed with CB exhibited a considerably greater Younger’s modulus than the construction printed utilizing OAB (Fig. 2f). We imagine that the distinction within the tensile modulus might have resulted from the completely different levels of crosslinking homogeneity throughout the struts, as proven in Fig. 2c. For the Gelma struts crosslinked with the OAB course of, the externally utilized tensile stress may very well be extremely focused on the comparatively low crosslinked area of the struts, inducing a decrease resistance of the tensile drive in contrast with that for the homogeneously crosslinked Gelma struts processed with CB.
To find out the suitable vary of printing parameters (UV dose and processing temperature) below a set Gelma weight fraction (5 wt%) and printing situations (nozzle dimension = 700 μm, nozzle shifting velocity = 1 mm/s, and pneumatic stress = 120 ± 10 kPa), a single line take a look at was carried out.
First, we measured the rheological properties of the 5 wt% Gelma bioink throughout temperature and time sweeps below numerous UV doses (30, 90, and 150 mJ/cm2). As anticipated, the Gelma bioink for the temperature sweep exhibited a typical sol–gel transition habits (Fig. 3a). As well as, the rise within the UV dose at a relentless temperature (10 °C) enhanced the crosslinking diploma of the Gelma bioink (Fig. 3b).
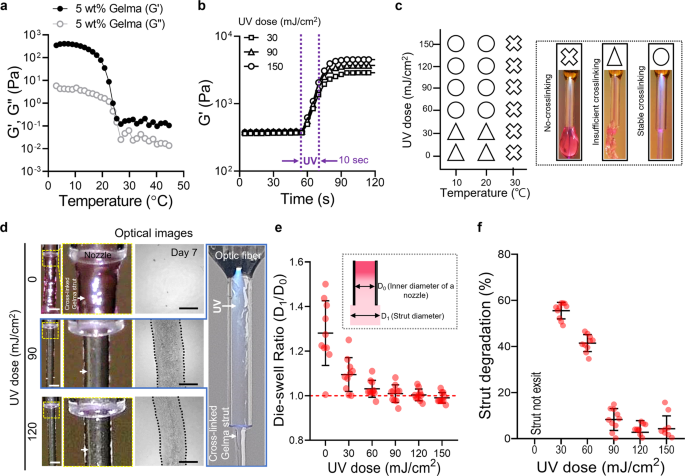
Rheological properties of 5 wt% Gelma obtained through (a) temperature sweep and (b) time sweep at numerous UV doses. c Course of diagram illustrating the impact of processing temperature and UV dose on the crosslinking diploma of printed Gelma struts (no-crosslinking, inadequate crosslinking, and steady crosslinking). d Optical photographs of the printed form of Gelma struts processed through OAB at numerous UV doses (0, 90, and 120 mJ/cm2) and optical photographs of the printed struts at 7 days of tradition. e Die-swelling ratios (diameter of printed strut/diameter of the nozzle) for numerous UV doses. f Degradation of printed Gelma struts decided utilizing the diameter distinction noticed earlier than and after 7 days of tradition. Scale bar, 1 mm (d—strut); 500 µm (d—cultured).
To watch the impact of the temperature of the Gelma bioink on the photo-crosslinking diploma, we chosen three typical processing temperatures: (1) gel area: 10 °C, (2) gel–sol transition area: 20 °C, and (3) sol area: 30 °C for numerous UV doses (0–150 mJ/cm2). For the sol area temperature (30 °C), steady photo-crosslinking of the printed Gelma strut was not obtained whatever the utilized UV dose (0–150 mJ/cm2), whereas for Gelma struts printed on the gel and gel–sol transition temperatures and with greater than 60 mJ/cm2 of UV doses, crosslinked Gelma struts had been stably shaped (Fig. 3c). The photo-crosslinking diploma for numerous sol–gel temperatures of Gelma bioink was according to that reported within the research carried out by Chansoria et al.26. In accordance with their analysis, the compressive modulus of Gelma bioink was extremely depending on not solely the UV-exposure situation but in addition on the photo-crosslinking temperature; the modulus elevated at decrease crosslinking temperatures (gel temperature, 10 °C) of Gelma. This consequence was obtained as a result of photo-crosslinking of the Gelma resolution at low temperature might induce a synergistic impact of tightly entangled polypeptide networks at low temperatures and their photo-crosslinking by covalent binding26.
As well as, to reveal the structural stability after photo-crosslinking/printing, we measured the die-swelling ratio (D1/D0, the place D0 and D1 point out the diameter of the internal nozzle and strut diameter on the nozzle tip, respectively) and the change within the cross-sectional space of the Gelma struts after 7 days within the PBS resolution. Determine 3d and Supplementary Fig. 2a depict optical photographs of the printed Gelma strut close to the printing nozzle at a set printing temperature (10 °C) and numerous UV doses (0–120 mJ/cm2). Owing to the low crosslinking diploma of Gelma uncovered to UV doses of 0 and 30 mJ/cm2, the swelling ratio was considerably greater than that of the printed Gelma crosslinked at greater UV doses (90 and 150 mJ/cm2) (Fig. 3e). Moreover, the circularity of the extruded strut elevated considerably till 120 mJ/cm2, nevertheless, the additional enhance in UV dose didn’t have an effect on the circularity (Supplementary Fig. 2b). Moreover, the circulate velocity (Supplementary Fig. 2c, d) and optic fiber to nozzle outlet distance (Supplementary Fig. 2e, f) had been investigated utilizing a set UV dose (120 mJ/cm2). As consequence, the circulate velocity had negligible results on the circularity of extruded struts, whereas the elevated distance between the optic fiber to nozzle distance had improved the circularity. An identical consequence was noticed for the degradation habits (=[A0 − A1]/A0, the place A0 and A1 denote the cross-sectional areas of in situ printed struts and the world of the strut at 7 days, respectively) of the Gelma struts (Fig. 3f). For UV doses under 90 mJ/cm2, strut degradation was extraordinarily speedy owing to inadequate crosslinking levels. Based mostly on the outcomes obtained utilizing strut formation and dimensions, we might choose acceptable settings for the UV dose (120 mJ/cm2).
After fixing UV dose, we investigated numerous water jacket temperatures (4, 10, and 20 °C), as proven in Supplementary Fig. 3a, b. Because of this, the homogeneity of the extruded strut measured utilizing circularity and floor smoothness confirmed that the strut processed with the comparatively low water jacket temperature (4 °C) indicated meaningfully greater circularity and smoother floor in comparison with these of the opposite temperatures. This may be attributed to the simultaneous UV crosslinking of Gelma at decrease temperatures can induce expressive structural stability27,28. As well as, when the temperature of the water jacket is about at 4 °C, the printing temperature was measured to be 10 °C. After fixing the UV dose and printing temperature, the impact of the burden fraction of Gelma on the crosslinking diploma was noticed. Supplementary Determine 4a, b illustrates the storage modulus for the three weight fractions (3, 5, and seven wt.%) of the Gelma bioink. As anticipated, the Gelma bioinks demonstrated an analogous sol–gel transition for the temperature sweep, and crosslinking below a set UV dose (120 mJ/cm2) and printing temperature (10 °C) was achieved. After using the UV- and stuck processing situations, we recorded the optical photographs and measured the die-swelling ratio and degradation of the Gelma struts printed utilizing the OAB course of (Supplementary Fig. 4c–e). For all concentrations of the Gelma bioink, a die-swelling ratio of roughly 1 was noticed; nevertheless, inadequate crosslinking was noticed on the three wt% Gelma bioink (Supplementary Fig. 4e). Based mostly on the outcomes, we used a Gelma focus of 5 wt% and the next printing parameters: UV dose = 120 mJ/cm2 and printing temperature = 10 °C for additional analysis. As proven in Supplementary Fig. 5, the dispersion of UV mild contained in the nozzle utilizing the set parameters can evoke crosslinking within the core area of the extruded strut, whereas the shell area is crosslinked comparatively much less. Based mostly on these knowledge, we rigorously predict that the optimized parameters within the OAB system can enable the fabrication of multilayered 3D buildings.
Utility of the OAB course of to acquire numerous buildings
Just lately, shape-morphing hydrogels or 4D biofabrication methods have been gaining consideration owing to the enabling of biomimicry and complicated microarchitecture formation29,30,31. To increase the OAB course of to the 4D fabrication of varied complicated organic architectures, we connected two optical fibers (optical fiber-1 and optical fiber-2) in a nozzle, proven within the schematic picture of Fig. 4a. Within the picture, because the optical fiber-1 was turned on, the uneven photo-crosslinking of the printed Gelma construction will be attained. Generally, excessive elastic property (≈excessive crosslinking diploma) induced in low water swelling32, in order that the diploma of uneven crosslinking of the hydrogel appears to find out the route of hydrogel curving. Determine 4b describes the curving mechanism intimately, and the optical picture of the Gelma strut that was fabricated utilizing the uneven photo-crosslinking course of can point out the uneven crosslinking of the strut. Determine 4c, d present the curving means of the Gelma struts relying on using the optics fibers. When uncovered to water, the asymmetrically photo-crosslinked Gelma struts initiated the completely different swelling charge, and various swelling behaviors occurred on the cross-sectional route of the strut because of the completely different crosslinking diploma, forming spiral and s-shaped buildings. Based mostly on the demonstrated proof-of-concept of the 4D fabrication, we rigorously predict that the OAB system will be utilized in numerous organic purposes comparable to blood vessel formation or nerve grafts.
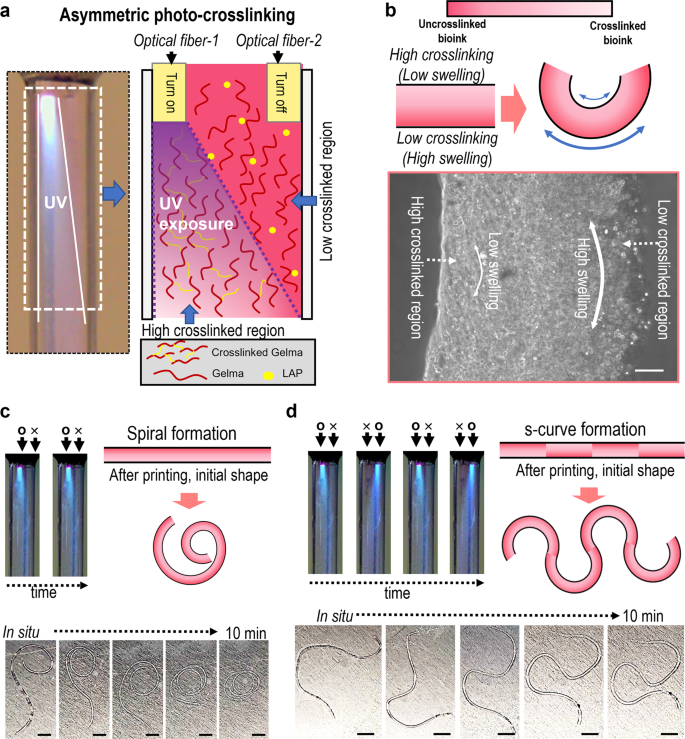
a Optical and schematic of uneven photo-crosslinking utilizing two optical fibers. b Form-morphing mechanism of the Gelma strut and an optical picture of the fabricated Gelma strut. Curving means (c spiral and d ‘s’-curve formation) of the Gelma struts relying on uneven crosslinking of Gelma struts. Scale bar, 100 µm (b); 5 mm (c, d).
As acknowledged within the earlier part, one of many benefits of the OAB course of is that photo-crosslinkable bioinks will be crosslinked throughout the nozzle, whatever the transparency of the nozzle materials and nozzle complexity. Three completely different nozzles (glass nozzle, opaque plastic nozzle, and squeezed metal nozzle) had been used to reveal the feasibility of the OAB course of, impartial of the particular nozzle design. As illustrated in Fig. 5a, a glass nozzle was used to extrude the Gelma bioink, and the Gelma struts printed with the CB and OAB processes below the beforehand set UV and printing situations demonstrated a very related cylindrical form (Fig. 5b). Nevertheless, when the OAB course of was employed utilizing the ellipsoidal metal nozzle proven in Fig. 5c, a Gelma strut with a form that was extra just like the form of the metal cross-sectional nozzle than the strut form printed with the CB course of was obtained (Fig. 5c, d).
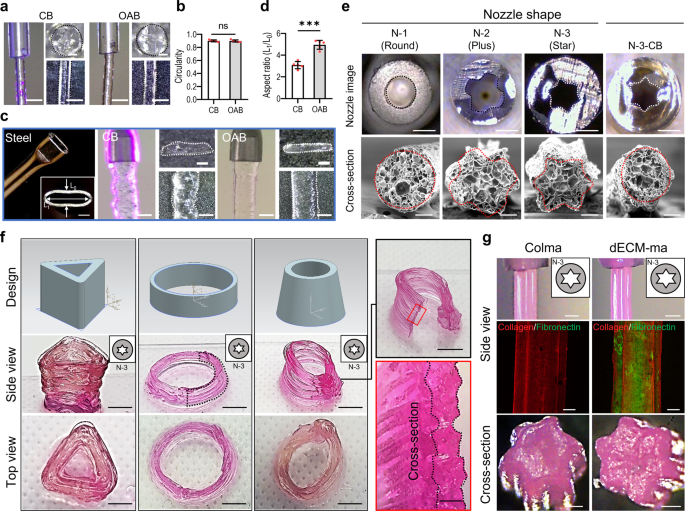
a Floor and cross-sectional optical photographs of the printed Gelma struts processed through CB and OAB (UV dose = 120 mJ/cm2) and b circularity of the cross-sectional photographs. c, d A metal nozzle with an ellipsoidal cross-sectional form, printed Gelma struts processed through CB and OAB, and the side ratio (size of the lengthy axis (L1)/size of the brief axis (L0) of the printed strut) decided utilizing the cross-sectional optical photographs of the printed struts. e Optical photographs of three plastic nozzles with completely different cross-sectional shapes (N-1: spherical, N-2: plus, N-3: star) and printed cross-sectional Gelma shapes fabricated utilizing every plastic nozzle and the OAB course of. N-3-CB signifies the cross-sectional optical picture of Gelma processed with the plastic nozzle form N-3 however crosslinked through the CB course of, not the OAB course of. f Optical photographs exhibiting numerous Gelma 3D buildings utilizing the N-3 nozzle and OAB course of and (g) optical and fluorescence (collagen: crimson and fibronectin: inexperienced) photographs of the floor and cross-sectional Colma and dECM-ma struts fabricated utilizing the N-3 plastic nozzle and OAB course of. All knowledge are introduced as imply ± SD. The p values had been calculated by scholar’s t-test (n = 3/group; *p < 0.05; **p < 0.01; ***p < 0.001). Scale bar, 1 mm (a, c-nozzle and strut,); 200 µm (a, c—strut cross-section, e—SEM, g—fluorescence and cross-section); 500 µm (c, e—nozzle cross-section, f—cross-section, g—nozzle optical photographs); 5 mm (f—facet view and prime view).
As well as, three opaque plastic nozzles with completely different cross-sectional shapes [round (N-1), plus-shaped (N-2), and star-shaped (N-3)] had been used to print the Gelma bioink utilizing the OAB course of, as proven in Fig. 5e. The cross-sectional photographs of the Gelma struts printed with the OAB course of exhibit shapes just like these of the printing nozzles, whereas the Gelma strut (N-3-CB) printed with the N-3 nozzle and CB course of doesn’t exhibit a form just like that of the printing nozzle (cross-sectional star-shaped). Moreover, Supplementary Fig. 6 depicts optical photographs of printed Gelma strut with N-2 and N-3 nozzles at numerous UV doses (0–150 mJ/cm2). To indicate the 3D Gelma shapes fabricated utilizing the OAB course of and N-3 nozzle, we fabricated the shapes with three completely different buildings, proven in Fig. 5f.
To increase the feasibility of the OAB course of to completely different methacrylated hydrogels, a methacrylated collagen (Colma) (methacrylation diploma: 79.3 ± 1.6%) and dECM methacrylate (dECM-ma, methacrylation diploma: 76.0 ± 2.4%) that was derived from the skeletal muscle tissue had been used. Schematic of decellularization/methacrylation processes utilizing porcine muscle tissue was described (Supplementary Fig. 7a), and DNA contents and collagen, elastin, and GAG contents for the muscle tissue, Colma and dECM-ma had been proven (Supplementary Fig. 7b–e). Determine 5g confirmed the floor and cross-sectional optical and fluorescence (collagen: crimson, fibronectin: inexperienced) photographs of the printed struts by utilizing the N-3 nozzle and OAB course of. The micro-grooved floor sample was clearly noticed within the Colma and dECM-ma struts.
Utility of the fabricated cell-laden struts to muscle tissue regeneration
By adopting the next printing parameters: UV dose = 120 mJ/cm2, temperature = 10 °C, printing stress = 120 ± 10 kPa, nozzle shifting velocity = 1 mm/s, cell-laden Gelma filaments (5 wt% Gelma, C2C12 density of 1 × 107 cells/mL) had been fabricated utilizing the CB and OAB-N-3 (nozzle form: N-3) processes. As illustrated in Fig. 6a, the floor and cross-sectional optical photographs of the cell-laden Gelma struts exhibit spherical and star shapes for the struts processed with CB and OAB-N-3, respectively. The stay (inexperienced)/lifeless (crimson) photographs and cell-viability (CB = 92.5 ± 2.2% and OAB-N-3 = 91.9 ± 1.8%) of the struts at 1 and three days of cell tradition revealed that the processes, together with every photo-crosslinking process, had been secure for the laden cells (Fig. 6b, c).
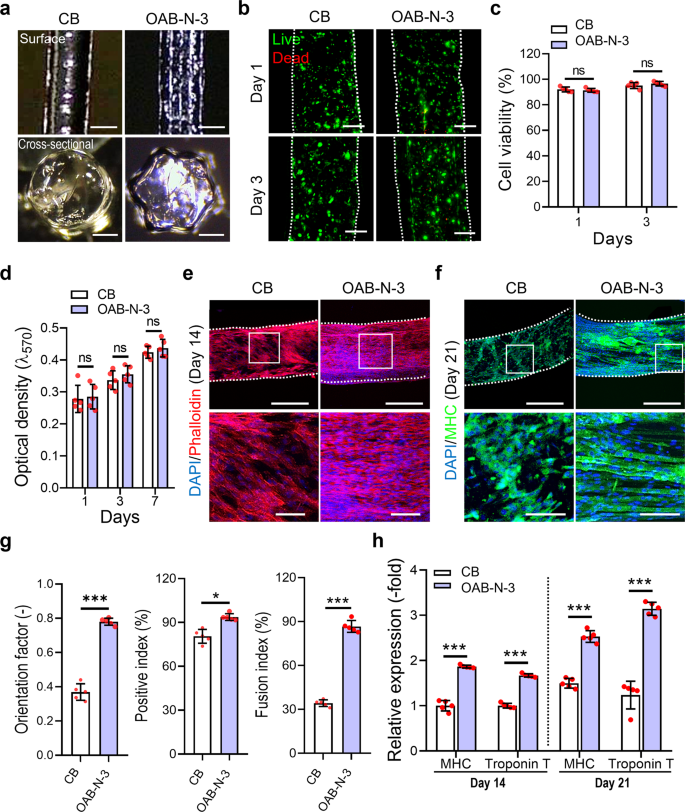
a Optical floor and cross-sectional photographs of the Gelma struts processed utilizing CB and OAB processes with the N-3 nozzle. b Dwell (inexperienced)/lifeless (crimson) at 1 and three days of tradition and (c) cell viability for every Gelma strut. d Cell proliferation decided utilizing the MTT assay for the Gelma struts. e DAPI (blue)/phalloidin (crimson) at 14 days of tradition and (f) DAPI/MHC (inexperienced) at 21 days of tradition (white field point out magnified area). g MHC orientation issue, optimistic index, and fusion index for the C2C12-laden Gelma struts at 21 days of cell tradition. h Relative gene expression of MHC and Troponin T in CB and OAB-N-3 buildings at 14 and 21 days of cell tradition. All knowledge are introduced as imply ± SD. The p values had been calculated by scholar’s t-test (n = 5/group; *p < 0.05; **p < 0.01; ***p < 0.001). Scale bar, 500 µm (a—floor, e, f—prime panel); 200 µm (a—cross-section, b); 100 µm (e, f—backside panel).
As well as, we measured cell proliferation utilizing the MTT assay for the struts and found that the proliferation charge was not considerably completely different between the struts. To watch the cell morphology of the Gelma struts, DAPI (blue)/phalloidin (crimson) photographs had been captured after 14 days of cell tradition. Within the recorded photographs, uniaxially aligned F-actin was noticed within the Gelma strut with a grooved floor sample fabricated utilizing the OAB-N-3 course of, whereas within the strut printed with the CB course of, an analogous uniaxial alignment of F-actin was not achieved (Fig. 6e). Moreover, DAPI/MHC (inexperienced) photographs at 21 days of cell tradition exhibited well-aligned and accelerated MHCs, decided by the orientation issue (=[90 − φ]/90, the place φ = full width at half most in an alignment distribution), and extra optimistic index/fusion index values had been noticed within the Gelma struts processed with OAB-N-3 than within the struts processed with CB (Fig. 6f, g).
To watch the myogenic differentiation of the printed Gelma struts, myogenic gene expression (MHC and troponin T) was noticed at 14 and 21 days of cell tradition (Fig. 6h). The myogenic genes had been upregulated within the Gelma group processed with OAB-N-3; this was owing to the topographical cue (groove form) contained in the nozzle, which was specifically designed to induce cell alignment. These outcomes are in good settlement with the outcomes of a number of research which have demonstrated that numerous scaffolds with uniaxial topographical patterns can modify the alignment of myoblasts and even myotube formation33,34,35. Herein, immortalized C2C12 cells had been used to judge the in vitro mobile actions. Nevertheless, the in vitro outcomes might differ when utilizing major autologous or allogenic cells.
In vivo research
The hASC-laden Gelma struts fabricated utilizing the CB, OAB-N-1 (nozzle form: N-1), and OAB-N-3 (nozzle form: N-3) processes had been utilized to VML tissue regeneration, which was obtained by excising 40% of an unique TA muscle in a mouse mannequin36. On this evaluation, we used hASCs to assist the regeneration of faulty VML37,38 as a result of the differentiation of hASCs into a number of cell lineages, together with a myogenic lineage, will be induced by numerous chemical and bodily stimuli39. As illustrated in Fig. 7a, the fabricated hASCs-laden Gelma constructs (cell density = 1 × 107 cells/mL, dimension = 2 × 4 × 1.6 mm3) (CB, OAB-N-1, and OAB-N-3) had been utilized within the defected muscle area. As anticipated, the hASCs had been alive in all three buildings, and the cells laden within the OAB-N-3 group had been nicely aligned within the printing route in contrast with these in CB and OAB-N-1 (Fig. 7a–c).
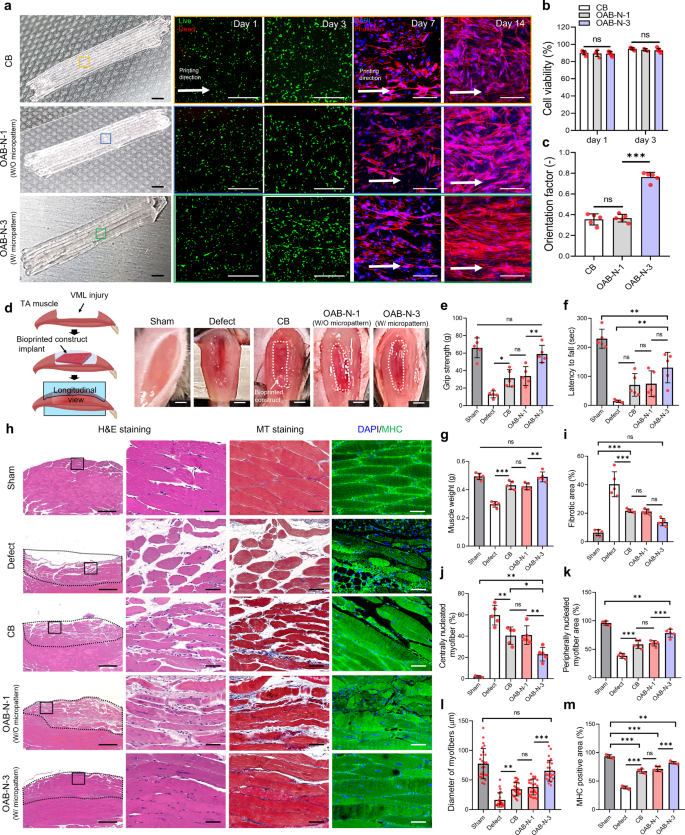
a Optical photographs, stay/lifeless at day 1 and three, and DAPI/phalloidin photographs at 7 and 14 days of CB and OAB muscle constructs (OAB-N-1 and OAB-N-3) laden with human adipose stem cells processed with N-1 and N-3 nozzles, respectively. b Cell-viability of the muscle constructs at 1 and three days of cell tradition (n = 5 per group, 4 random fields in every pattern) and (c) orientation issue utilizing F-actin at 14 days of cell tradition (n = 5 per group, 4 random fields in every pattern). d Gross look of the constructs implanted within the TA muscle defect. Quantified (e) grip energy, (f) latency to fall take a look at, and (g) muscle weight after 4 weeks implantation (n = 5 per animals, three repeated measurements per pattern). h H&E (purple: nuclei, pink: cytoplasm), MT (crimson: muscle fiber, blue: collagen), and DAPI/MHC (inexperienced) of transplanted websites, (i) fibrotic space (%) (n = 5 per group, 4 random fields in every pattern), (j) centrally nucleated myofiber (%) (n = 5 per group, 4 random fields in every pattern), (ok) peripherally nucleated myofiber space (%) (n = 5 per group, 4 random fields in every pattern), (l) diameter of myofibers (μm) (n = 25 per group, 4 random fields in every pattern), and (m) MHC-positive space (%) (n = 5 per group, 4 random fields in every pattern) for sham, defect solely, CB, OAB-N-1, and OAB-N-3 samples. All knowledge are introduced as imply ± SD. The p values had been calculated by scholar’s t-test (b, c), and one-way ANOVA with Tukey’s put up hoc take a look at (e–m) (NS statistical nonsignificance, *p < 0.05; **p < 0.01; ***p < 0.001). Scale bar, 2 mm (a—optical, d); 500 µm (a—stay/lifeless, h–H&E); 100 µm (a—DAPI/phalloidin); 50 µm (h—H&E magnified, MT, DAPI/MHC).
To evaluate the effectivity of VML muscle regeneration, 5 teams: (1) sham (no defect), (2) solely defect, (3) CB, (4) OAB-N-1, and (5) OAB-N-3 group had been implanted within the mouse mannequin (Fig. 7d). Analysis of grip energy and latency to fall is a dependable evaluation of muscle regenerative efficacy14,40,41,42. As such, the outcomes of the grip energy take a look at and the autumn latency take a look at in mice 4 weeks after implantation of the constructs are proven in Fig. 7e–g. The OAB-N-3 group confirmed a considerably greater grip energy than the opposite teams, and related grip energy to the sham (Fig. 7e). Nevertheless, the autumn latency time confirmed a comparatively longer latency to fall in comparison with the opposite teams however was considerably decrease than that of the sham group (Fig. 7f). We attribute these findings to the denervation of the muscle fibers after the VML defect, leading to purposeful impairment42,43,44. To evaluate this impact, the muscle sections harvested had been stained with acetylcholine receptors (AchR) (Supplementary Fig. 8a). Considerably greater expressions of AchR had been noticed within the mice that acquired OAB-N-3 in comparison with different teams (Supplementary Fig. 8b). As well as, the muscle weight of the OAB-N-3 group was similar to that of the sham group (Fig. 7g). Determine 7h illustrates the longitudinal histological outcomes of H&E, MT staining, and DAPI/MHC, and Supplementary Fig. 9 exhibits the cross-sectional H&E staining. The fibrotic areas, centrally nucleated myofiber, peripherally nucleated myofiber space, diameter of myofibers, and MHC-positive space had been measured, and the outcomes are introduced in Fig. 7i–m. As illustrated within the outcomes, extra environment friendly muscle regeneration and a low diploma of fibrosis (blue space in MT staining) had been detected within the OAB-N-3 group in contrast with the corresponding values within the CB and OAB-N-1 teams, demonstrating that the topographical cues, attributable to the groove of the nozzle used within the OAB-N-3 course of. Moreover, the centrally nucleated muscle fibers on the muscle sections harvested from the mice that had been processed with OAB-N-3 had been significantly decrease in comparison with these of defect, CB, and OAB-N-142,45,46. Based mostly on these outcomes, we rigorously predict that OAB-N-3 bioconstruct can induce more practical muscle regeneration than that within the CB and OAB-N-1 teams with none topographical cues.
Extra apparently, myofibers shaped within the implanted constructs had been originated from the printed hASCs within the CB, OAB-N-1, and OAB-N-3, respectively, as confirmed by double-immunofluorescence for MHC/MRPL11 and MHC/HLA-A at 4 weeks after implantation (Fig. 8a). Particularly, it may be noticed that the OAB-N-3 group has considerably greater MRPL11 and HLA-A optimistic muscle fibers than different teams (CB and OAB-N-1) (Fig. 8b, c). Based mostly on the histological outcomes, we are able to conclude that muscle regeneration utilizing the cell-laden Gelma assemble processed utilizing the OAB-N-3 course of will be accelerated in contrast with that utilizing the Gelma assemble processed utilizing the CB and OAB-N-1 course of. Nevertheless, regardless of these favorable muscle regenerative outcomes concerning the proposed assemble (OAB-N-3), it’s price stating that owing to the small defect sizes of the mouse VML mannequin, the medical relevance is missing.
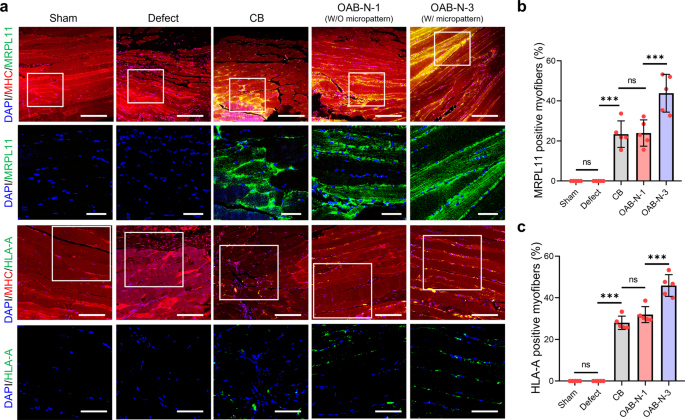
a Double-immunofluorescence photographs of DAPI (blue)/MHC (crimson)/MRPL 11 (inexperienced) and DAPI (blue)/MHC (crimson)/HLA-A (inexperienced). Quantified (b) MRPL11 and (c) HLA-A optimistic myofiber (%) (n = 5 per group, 4 random fields in every pattern). All knowledge are introduced as imply ± SD. The p values had been calculated by one-way ANOVA with Tukey’s put up hoc take a look at (NS = statistical nonsignificance, *p < 0.05; **p < 0.01; ***p < 0.001). Scale bar, 200 µm (a—DAPI/MHC/MRPL11); 50 µm (a—DAPI/MRPL11, DAPI/HLA-A); 100 µm (a—DAPI/MHC/HLA-A).
On this research, we developed a novel bioprinting course of supplemented with an optical fiber-assisted photo-crosslinking technique to manufacture cell-laden filaments with micropatterned surfaces for tissue engineering purposes. To acquire biofunctional cell-laden struts with a topographical cue, acceptable printing parameters, comparable to UV dose and printing temperature, had been rigorously chosen. To reveal the feasibility of the bioprinting course of, C2C12 myoblasts and hASCs had been used to watch in vitro myogenic actions and in vivo volumetric muscle defects, respectively, in a mouse mannequin. Because of the biomimetic phenotype on the cell-laden Gelma constructs processed with OAB-N-3, improved in vivo VML restore was noticed in comparison with bioconstructs printed through the standard printing processes. Based mostly on these outcomes, we imagine that the newly designed bioprinting course of mixed with optical fiber-assisted crosslinking can function a promising strategy for fabricating biofunctional cell-laden constructs for software in numerous tissue regenerations.